Abstract
Introduction:
Direct oral anticoagulants (DOACs) are being increasingly used due to their improved safety and efficacy as compared with warfarin. However, unlike warfarin, less is known regarding long-term and large-scale side effects of DOACs. In order to better evaluate the rates of DOAC-related adverse events on a population level we examined the rate of adverse events reported to the U.S. Food & Drug Administration (FDA) for three commonly used DOACs and warfarin.
Methods:
We evaluated the FDA Adverse Event Reporting System (FAERS) database, which compiles drug-related adverse events reported to the FDA from 1969 onwards. The safety profiles of warfarin and three DOACs were assessed by comparing adverse events per outpatient prescription and with proportional reporting ratios (PRR). Adverse event data for each drug (warfarin, dabigatran, rivaroxaban, apixaban) was gathered from FAERS. The generic name of each drug was used, and in the case of dabigatran and warfarin, dabigatran etexilate mesylate and warfarin sodium respectively were used to query the database. Adverse events data was filtered by including only those reported by healthcare professionals in the U.S. Specific adverse events were chosen apriori based on hypothesized potential hemorrhagic and thrombotic complications. Numbers of U.S. outpatient prescriptions were gathered from the ClinCalc DrugStats database using information from the Medical Expenditure Panel Survey (MEPS). Only adverse events data from years in which there was also prescription data were included in the study. PRR with 95% confidence interval was used to compare rates of DOAC adverse events, with warfarin being excluded to avoid the new-drug effect. A PRR of 2 or greater was considered significant.
Results:
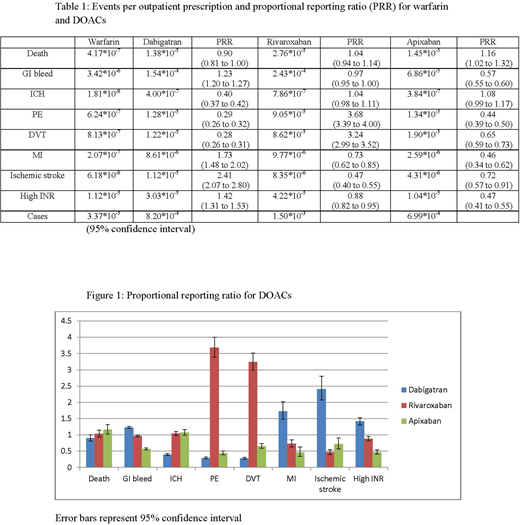
As shown in Table 1, warfarin had the fewest reported adverse events per prescription for all categories save elevated international normalized ratio. Rivaroxaban had the highest proportion of reported adverse events, most notably the rate for breakthrough venous thromboembolism (VTE) was higher than for other DOACs. Dabigatran had the highest reported rated of ischemic stroke. When the DOAC data was analyzed using PRR, as seen in Figure 1, less difference was observed. Reported rates of pulmonary embolism (PE) and deep-vein thrombosis (DVT) were again higher with rivaroxaban while dabigatran again showed higher than expected rates of ischemic stroke and a slightly higher risk of myocardial infarction. Apixaban did not show higher than expected rates in any category. Reported rates of mortality did not significantly differ among the drugs.
Conclusion:
Our analysis found that among DOACs rates of breakthrough VTE were significantly higher with rivaroxaban, and that reported rates of ischemic stroke were significantly higher with dabigatran. While no DOACs have been compared against each other in prospective trials, post-marketing reports have suggested that different safety profiles exist among DOACs, a finding reiterated by our analysis. The significantly higher rate of VTE reported with rivaroxaban as compared to other DOACs has not previously been described to the best of our knowledge and deserves further analysis. The two methods of comparing the anticoagulants - reported event rate per prescription and PRR - show slightly different results, but the adverse findings reported with rivaroxaban and dabigatran remain consistent. Limitations of this study include the reliance on the accurate reporting of adverse events and reliability of the MEPS data, possible duplications in the FAERS database, and the importance of not conflating correlation with causation as it relates to these events. Considering both event rate per prescription and PRR, apixaban seems to offer the best safety profile of DOACs according to the FAERS data. Further study is needed to better characterize the comparative safety profiles of DOACs.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal